Kontrol BioCloud®
- Provides an update on the timing of the next stage of testing for the Kontrol BioCloud® analyzer
- Kontrol has designed BioCloud with a replaceable detection mechanism. Kontrol will seek to patent the detection mechanism following the next stage of testing
- Company will also analyze the best method of replacing and disposing of the detection mechanism after it comes into contact with COVID-19
- The detection mechanism has been built to be an ongoing replaceable component of the BioCloud technology
TORONTO, ON / August 19, 2020 / Kontrol Energy Corp. (CSE:KNR)(OTCQB:KNRLF)(FSE:1K8) (“Kontrol” or “Company“) is pleased to provide an update on the timing of the next stage of testing for the Kontrol BioCloud® analyzer (“BioCloud“).
For more than a decade, through its operating subsidiaries, Kontrol has been providing air quality testing and continuous emission monitoring across hundreds of industrial facilities in Canada and the USA. BioCloud represents an extension and further innovation of an existing air quality technology and solution platform which Kontrol currently provides to its customers.
“We are drawing on our extensive experience in air quality and emission monitoring for specific particulate analysis and measurement and using similar technology to detect for COVID-19 in the air,” says Paul Ghezzi, CEO of Kontrol. “Over the past 6 months, we have secured government funding, established partnerships with virology experts, and developed a proprietary COVID-19 detection chamber. Our initial lab results and interest from potential customers and prospects give us the confidence that we are developing a solution which the market demands. We look forward to providing more details as we continue BioCloud’s development.”
Next Stage of Testing
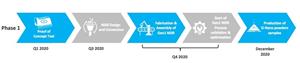
Live COVID-19 virus testing will commence on August 19th, 2020 and is expected to continue until the end of the month. The testing will focus on the capability of the working BioCloud prototype to sample the COVID-19 virus in aerosol form and seek to establish the lower detection limits that can be achieved. Kontrol will publish the results following the testing.
Detection Mechanism
Kontrol has designed BioCloud with a replaceable detection mechanism. Kontrol will seek to patent the detection mechanism following the next stage of testing. Kontrol will also analyze the best method of replacing and disposing of the detection mechanism after it comes into contact with COVID-19. The detection mechanism has been built to be an ongoing replaceable component of the BioCloud technology.
“BioCloud will seek to sample the air over-time on a continuous basis, which is similar to how we currently test for air quality in industrial facilities. Applications for BioCloud will be focused initially on classrooms, hospitals, long-term care homes, and mass transportation.” said Gary Saunders, Kontrol’s Vice President.
Pre-Commercialization Prototypes
Kontrol has built one operating prototype and is in the process of completing four additional pre-commercialization prototypes. These prototypes will be made available for lab testing and also provide Kontrol with greater certainty for developing its supply chain for parts and materials for potential future commercialization. The additional four prototypes will be completed prior to August 31st, 2020.
The Company is not making any express or implied claims that its product has the ability to eliminate, cure or contain the Covid-19 (or SARS-2 Coronavirus) at this time.
About Kontrol Energy
Kontrol Energy Corp. (CSE:KNR)(OTCQB:KNRLF)(FSE:1K8) is a leader in the energy efficiency sector through IoT, Cloud and SaaS technology. With a disciplined mergers and acquisition strategy, combined with organic growth, Kontrol Energy Corp. provides market-based energy solutions to our customers designed to reduce their overall cost of energy while providing a corresponding reduction in greenhouse gas (GHG) emissions.
 |  |
Additional information about Kontrol Energy Corp. can be found on its website at www.kontrolenergy.com and by reviewing its profile on SEDAR at www.sedar.com.
For further information, contact:
Paul Ghezzi, Chief Executive Officer
[email protected] or [email protected]
Kontrol Energy Corp.,
180 Jardin Drive, Unit 9, Vaughan, ON L4K 1X8
Tel: 905.766.0400, Toll free: 1.844.566.8123
Neither IIROC nor any stock exchange or other securities regulatory authority accepts responsibility for the adequacy or accuracy of this release.
Forward-Looking Statements
This news release contains “forward-looking information” within the meaning of applicable securities laws. All statements contained herein that are not clearly historical in nature may constitute forward-looking information. In some cases, forward-looking information can be identified by words or phrases such as “may”, “will”, “expect”, “likely”, “should”, “would”, “plan”, “anticipate”, “intend”, “potential”, “proposed”, “estimate”, “believe” or the negative of these terms, or other similar words, expressions and grammatical variations thereof, or statements that certain events or conditions “may” or “will” happen, or by discussions of strategy.
Where the Company expresses or implies an expectation or belief as to future events or results, such expectation or belief is based on assumptions made in good faith and believed to have a reasonable basis. Such assumptions include, without limitation, that sufficient capital will be available to the Company and that technology will be as effective as anticipated.
However, forward-looking statements are subject to risks, uncertainties, and other factors, which could cause actual results to differ materially from future results expressed, projected, or implied by such forward-looking statements. Such risks include, but are not limited to, that sufficient capital and financing cannot be obtained on reasonable terms, or at all, that technologies will not prove as effective as expected, that customers and potential customers will not be as accepting of the Company’s product and service offering as expected, and government and regulatory factors impacting the energy conservation industry. In particular, successful development and commercialization of the Kontrol BioCloud Analyzer are subject to the risk that the Kontrol BioCloud Analyzer may not prove to be successful in detecting the virus that causes COVID-19 effectively or at all, uncertainty of timing or availability of any regulatory approvals and Kontrol’s lack of track record in developing products for medical applications.
Accordingly, undue reliance should not be placed on forward-looking statements and the forward-looking statements contained in this press release are expressly qualified in their entirety by this cautionary statement. The forward-looking statements contained herein are made as at the date hereof and are based on the beliefs, estimates, expectations, and opinions of management on such date. Kontrol does not undertake any obligation to update publicly or revise any such forward-looking statements or any forward-looking statements contained in any other documents whether as a result of new information, future events or otherwise or to explain any material difference between subsequent actual events and such forward-looking information, except as required under applicable securities law. Readers are cautioned to consider these and other factors, uncertainties, and potential events carefully and not to put undue reliance on forward-looking information.
SOURCE: Kontrol Energy Corp.