Gold is trading at historic highs, with analysts projecting continued strength into 2026 and beyond. Yet for junior and senior developers, the traditional path to mining gold still comes with major challenges: mining is expensive, slow, and often environmentally and socially destructive.
There are, however, high barriers to entry for investors. The opportunity to benefit from these historic highs in the price of gold are made difficult by the limited supply, substantial costs of physical storage, security, auditability, insurance, shifts towards more sustainable investments and significant premiums over the spot price. While modern financial instruments like Exchange Traded Funds (ETFs) and digital gold tokens have improved accessibility, traditional, high-trust forms of investment remain restricted by high capital requirements and limited, in many regions, to high-net-worth individuals or institutional players.
That tension is exactly where nGRND believes the next evolution of gold ownership begins.
In a recent interview with AGORACOM founder George Tsiolis, Professor Lisa Wilson, the CEO of nGRND Inc., outlined a new model that challenges centuries of convention: what if gold could create economic value without ever being mined?
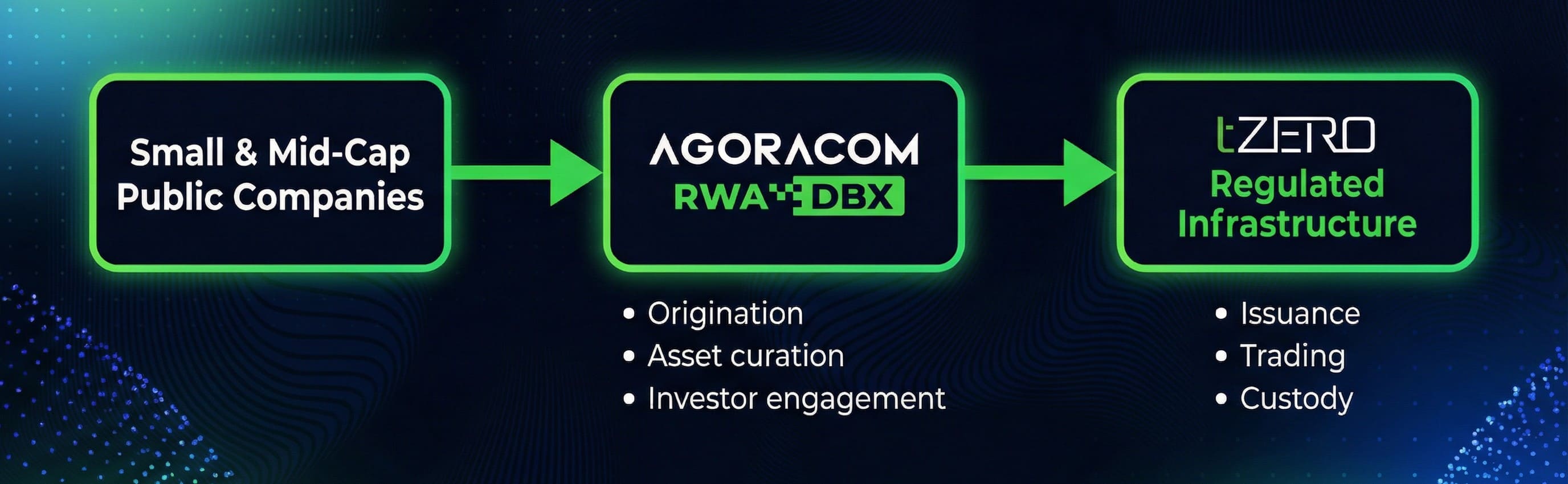
Rather than extracting gold, nGRND focuses on acquiring the long-term rights to sites with known verified in-ground gold Mineral Resources and enables their monetisation through alternative land usage from avoided mining. nGRND sponsor a fully regulated and licensed issuer to generate real world 100% asset-backed ownership structures – allowing the verified gold to remain untouched beneath the surface in-ground.
“We go out to globally to find known, verified Mineral Resources” Professor Wilson explained, “procure those assets for a minimum of 30 years, and create an alternative way of valuing and monetising that gold in-ground.”
The outcome is a new category of sustainable gold ownership designed for a world where investors are increasingly expecting hard-asset stability and environmental accountability.
Unlocking the value from gold that is currently uneconomical to mine.
One of the most striking points in the interview is the sheer scale of verified gold that already exists in the ground—but remains economically stranded.
Wilson noted that there are nearly six billion ounces of known in-situ gold resources globally. Much of it is held by junior developers and exploration companies, many based in Canada. Yet these ounces often remain unmonetised because advancing a project to production is an enormous undertaking and in many cases currently uneconomical.
Key barriers include:
- Long development timelines, often 7 to 20 years
- Massive capital requirements to build a mine
- Permitting complexity and increasingly difficult environmental approvals
- Sites that may be currently uneconomical or environmentally sensitive
For many resource owners, this leaves gold trapped in a frustrating limbo: valuable in theory, but difficult to turn into an economic reality.
nGRND’s proposition is to change that equation.
nGRND Inc. offers an alternative monetisation pathway that keeps the gold in-situ while still recognising its value proposition.
A new value proposition for verified resource owners
Traditionally, junior development companies seeking liquidity or capital for further development are often forced to sell assets at steep discounts to major producers or obtain royalty and streaming agreements.
Professor Wilson described conventional in-ground transactions as frequently yielding at the top end only $60 to $90 per ounce, and often sometimes far less.
nGRND offers something materially different.
According to Professor Wilson, the company aims to provide resource owners roughly 250% more than traditional in-situ pricing, which is approximately $210 to $220 per ounce at current gold levels – while allowing them to retain land ownership and avoid decades of uncertainty.
This liquidity can then be used to:
- Strengthen balance sheets
- Continue exploration and resource classification upgrading
- Reduce dependence on dilutive financing
- Potentially return value to shareholders
In Professor Wilson’s words this gives junior developers “cash on their books” and an option beyond “crumbs” offered by the traditional system.
Gold as a store of value – whilst in remains underground
A natural question arises: If the gold stays underground, how can it still function as an asset?
Professor Wilson’s answer is simple: Gold is gold, whether it is above ground or still in the earth. It has the same properties in-situ as extracted. The only thing you cannot do is wear it!
Lisa offered a modern analogy:
“Every time you look at your digital bank account, you don’t physically see the cash – but you still recognise it as value.”
In nGRND’s view, verified in-ground gold can serve the same purpose as physical gold stored in a vault, as a scarce, real store of value, but preserved rather than extracted.
Regulated real-world asset ownership through Tokinvest
A defining feature of nGRND’s approach is its emphasis on verification and regulatory structure.
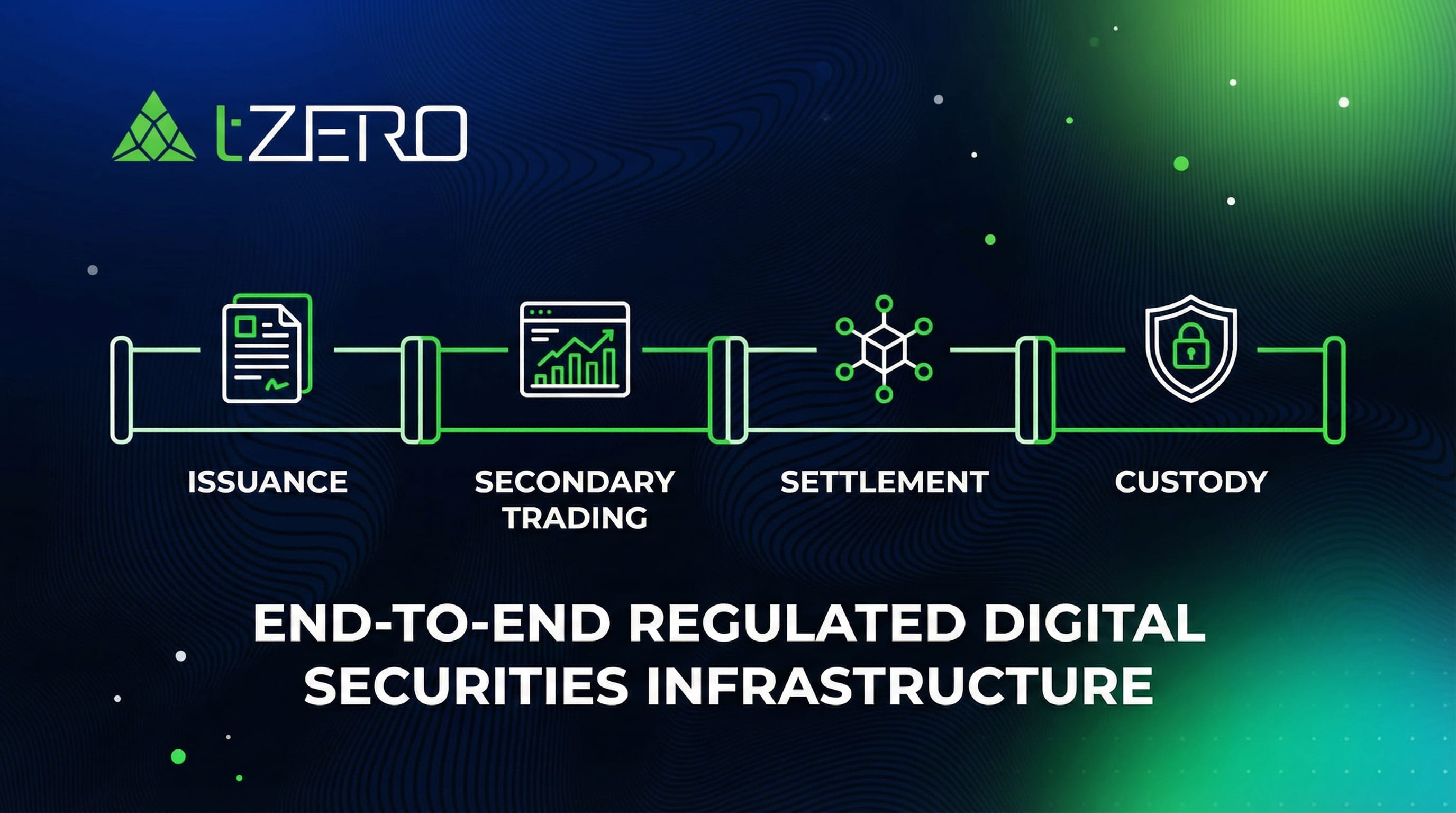
The company does not generate or issue tokens directly. Instead, it sponsors the development of a 100% asset-backed gold ownership structure generated, issued and sold by Tokinvest, a licensed and VARA-regulated entity in Dubai.
Professor Wilson stressed that this regulated architecture is part of what differentiates nGRND from earlier, unsuccessful attempts to digitise in-ground gold ownership.
Key safeguards include:
- Auditability, provenance and verification of the gold
- Resource verification must meet recognised standards such as NI 43-101, JORC, or SAMREC, or equivalent
- Tokens remain in an Token Generation Event (TGE) escrow pool until they are fully backed by contracted verified ounces obatined through nGRND Inc Site Programmes
- nGRND Gold Tokens are only released by Tokinvest when they are 100% backed by an equivalent verified gold resource
“No verification, no tokens,” Wilson emphasised.
This framework is designed to expand the potential investor base beyond retail participants to include volume trades by institutions already familiar with gold ETFs and regulated commodity products.
A parallel revenue engine: ESG and Carbon Programmes
nGRND’s model extends beyond gold.
By keeping gold in the ground – what Professor Wilson calls “avoided mining” nGRND Inc. enables alternative land-use programmes that can generate additional revenue independent of gold price movements.
These Site Programmes will have Carbon and ESG feasibility , invesatbility and origination conducted by independent partners CarbonPlanet and Foresteam. The Site Programmes will include:
- Carbon offset standards accrediting origination from greater than 540 methodologies
- Land rehabilitation and biodiversity restoration
- Renewable energy development
- Environmental restoration of brownfield sites
Crucially, Wilson explained that these programmes remain structurally separate from the gold-backed asset itself.
The gold ownership structure is treated as a commodity-backed asset, while ESG and carbon revenues form an independent dual distribution stream for nGRND Inc and nGRND Gold Token holders.
In Professor Wilson’s framing, this creates investment exposure to two uncorrelated commodity assets and themes:
- Gold as a store of value and traditional commodity
- Carbon and environmental assets as a rapidly emerging and high growth asset class
Early momentum and a 2026 rollout timeline
nGRND Inc has only just exited “stealth mode”, but Professor Wilson indicated that traction has materialised quickly and very strongly during its development and the weeks post.
“Before we even exited stealth mode, we had a signed LOI to take over a property,” Lisa said, adding that the company has already surpassed its internal 2029 mineral ounce pipeline targets.
The company expects the Token Generation Event by Tokinvest to occur around April 2026, with regulated professional and retail access to follow shortly thereafter through Tokinvest.
This rollout is structured, verification-gated, and designed to scale over time as additional site programmes are secured.
Why investors are paying attention now?
nGRND Inc. sits at the intersection of several powerful global forces:
- Rising gold demand in uncertain macro environments
- Increasing investor focus on sustainability and ESG accountability
- Institutional adoption of regulated digital asset infrastructure
- A growing need to separate resource value from environmental cost
In short, nGRND is positioning itself with the vision of being the world’s largest resource company who don’t mine!
The bottom line: A structural shift in how gold Is monetised
For centuries, gold ownership has depended on extraction.
nGRND is delivering something fundamentally different. Verified gold can be monetised, preserved, and owned sustainably – without the delays, costs, and environmental disruption of mining.
With long-term control of Site Programmes with known verified in-ground resources, regulated issuance through Tokinvest, and parallel ESG and carbon programme development through CarbonPlanet and Foresteam, nGRND is advancing a model that will reshape how the world thinks about natural wealth.
As Professor Wilson put it:
“Gold is gold. Whether it is above ground or in the ground, it has the same properties as a store of value. We have created a way to monetise it without destroying the land or waiting decades to build a mine.”
For investors watching the evolution of real-world asset ownership, sustainable finance, and gold’s role in the modern economy, nGRND is emerging as one of the most innovative and closely watched new entrants in the space.
DISCLAIMER AND DISCLOSURE
This record is published on behalf of the featured company or companies mentioned (Collectively “Clients”), which are paid clients of Agora Internet Relations Corp or AGORACOM Investor Relations Corp. (Collectively “AGORACOM”)