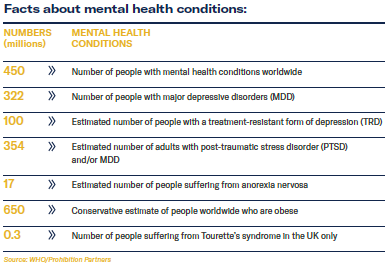
- Neuropharmacologist and Functional Medicine practitioner Dr Elisabeth Philipps tells MCN about the therapeutic benefits of CBD and talks about CBD regulation.
MCN speaks to Dr Philipps about the science behind the health benefits of CBD and the need for standardised regulation of CBD food supplements.
Dr Elisabeth Philipps is a neuropharmacologist and practitioner in Functional Medicine with over 18 years’ experience. She operates a thriving health consultancy in the UK specialising in CBD, brain and gut health; where she provides expert opinion for private clients, corporate business programmes, the national media, specialist healthcare publications and health companies.
How did you first become aware of the endocannabinoid system and the therapeutic benefits of cannabis?
I’m a neuropharmacologist by training, so I’ve been aware of the medicinal therapeutic benefits of cannabis for a number of years. The endocannabinoid system was only discovered in the 1990s so it’s actually a relatively recent system that we’ve known about in the body, but it’s only been in the last two to three years that my focus has become much more intense on the endocannabinoid system, medicinal cannabis and CBD oil supplements; because of the rise in profile of those products.
What are the potential dietary benefits of consuming CBD or hemp?
The two products are quite different: hemp is extract of the whole plant, so the dietary benefits of hemp normally come from the hemp seed – you could have the seeds ground or sprinkled on top of your food, for example; maybe hemp seed oil which makes a good salad dressing. Hemp oil extracts are rich in very beneficial omega-3 and omega-6 essential fatty acids; you also find things like protein and fibre as well as minerals and vitamins: magnesium and phosphorus, for example, are found within the hemp seeds themselves.
CBD is an even more refined extract from the hemp plant flowers rather than the seeds, so there’s not so much [in the way of] dietary benefit; but it contains the compounds such as the phytocannabinoids, and other compounds such as flavonoids and terpenes, which can confer health benefits; so you get the dietary benefits from hemp seeds and hemp seed oil and you get other health benefits from consuming CBD.
What kind of conditions and ailments do you find that people are using CBD for?
[There are] three primary areas that people find CBD works for them: the sleep area, to help improve the quality of sleep; people report being able to [get to] sleep more easily, stay asleep and feel more refreshed when they wake up. [The second area is] pain, which comes in many different forms – it can be nerve pain or neurogenic pain or inflammatory pain, so conditions such as arthritis, for example, or more acute conditions such as sporting injuries or general inflammatory injuries. People report taking CBD oil as helping to reduce the response to pain. What’s been very interesting in the study of CBD and phytocannabinoids in pain pathways is that not only is it blocking the pain pathways themselves in the spinal cord and brain, but also CBD acts within the parts of the brain that helps change our perception of pain. That’s really key, because it’s how we respond to the pain signals in our bodies that gives us the intensity of the experience; so CBD is an important component of a programme to help manage different types of pain.
The third area that I find CBD can really help is in mood, in particular [for patients who are] feeling anxious or stressed. CBD has this ability, through various pathways in the brain, to help balance the systems that help us feel better, to improve wellbeing, to improve our mood. CBD works at the neurotransmitter level, transmitting chemicals in the brain that send signals to help provide a balance. There are a number of studies coming in: they’re relatively small scale, but they’re looking at CBD oil for anxiety and for sleep and they have showed improvement in those areas; but the science is beginning to play catch-up now as well.
What is the science behind the effects of CBD on different symptoms?
CBD interacts with our own endocannabinoid system and I think that’s really key, because when you take a medicine it’s a very blunt tool: it either turns something on or turns something off; it blocks a response or it produces a response. CBD is lot more subtle than that within the body, because it works within the endocannabinoid system and that system is spread throughout our body – the endocannabinoid system is in the gut, the brain, in the immune cells, in the lungs, in the skin – so that’s why CBD can have such a diverse number of effects.
We produce our own cannabinoids within the body to stimulate and produce effects; and CBD helps balance the body’s production and the body’s ability to activate the endocannabinoid system through its own naturally produced mediators. There’s a lot of science showing how CBD acts within the endocannabinoid system and produces its responses.
What advice do you have for consumers when choosing a brand or product to ensure it is good quality and does not contain any unlisted ingredients?
This can be a very confusing area, because there are so many products that have hit the market. First of all, a CBD food supplement is legal in the UK if it contains less than 0.2% THC or tetrahydrocannabinol. That is the cannabinoid which causes the commonly associated high or buzz that you get from marijuana; it’s only present in hemp in very low levels anyway and it’s been stripped out of CBD oil. You need to ensure that you’re taking a legal product that contains less than 0.2% THC; and you can do that by asking the company for a certificate of analysis.
Every batch of CBD oil that a reputable company receives will have had a third-party laboratory testing [conducted] on that particular batch of oil, which will have a batch number so it can all be traced. That will show the breakdown of the different cannabinoids and the different constituents of that particular oil so you’ll be able to see not only that it contains the legal limit or less of THC, but the amount of CBD that it says on the label as well – that’s where the product can vary drastically, so you need to have a look at that certificate of analysis to show that it contains the CBD that you want. You will find some companies selling 0% THC products, which are very useful especially if you are drug tested for work or if you’re a professional athlete. The Banned Substances Control Group (BSCG) can test for THC and other banned substances and can provide a certificate to show that you don’t have any of those substances within your product. Reputable companies should have their certificates of analysis on their websites; or have a chat with them and they should provide that information for you.
How do you anticipate the market will evolve in over the next few years?
This is a very interesting time for CBD and medical cannabis: there’s obviously a lot going on and there’s a lot in the news. I think there needs to be more CBD regulation within the industry, because there’s a wide range of products on the market that differ in terms of their CBD content and what they purport to have in them; so there needs to be more CBD regulation, there’s no doubt about that. Equally [there is a question of] how that CBD regulation is brought in: the industry is playing catch-up in a way because the popularity of the product has grown exponentially in the last few years.
What we need to see is regulation, working with food standards agencies and [other regulators] at a government level. I think that will evolve over the next few months and years; and it’s going to be [because of] FourFive and other reputable companies that are working with the regulators, to help the industry in a way that protects it and still provides consumers with quality products.
Working on the assumption that further CBD regulation is likely to be introduced, do you expect this to impact patients using these products?
There is no doubt that the CBD regulation will change; and that it needs to protect the industry in terms of protection of products and protect consumers as well. I think it’s a case of companies and experts working with both agencies and the regulators: the impact on patients is our primary concern; and we are working towards avoiding any impact so there is seamless availability of quality products on the market. That is a goal that everyone is working to at the moment: every country is different and we’re just going to have to keep working together to make sure that high quality products [are going] to be readily available.
Do you anticipate Brexit having an impact, either positive or negative, on CBD regulation and medicinal cannabis regulation?
Brexit will potentially free up time and space for this topic to be higher up agendas. I believe that Brexit has possibly moved discussions of CBD and medical cannabis off the table or further down the list [of priorities]. I do think we’ll see more discussions happening in the next few weeks and months as Brexit resolves. At the moment we’re still working under European guidelines, in terms of the food supplement side at least, but it will be interesting to see how that pans out as we leave Europe; and as with many different areas of Brexit it’s still a bit of an unknown as to how EFTA’s [the European Free Trade Association] regulations and rules will affect the Food Standards Agency on the UK side. There will definitely be more discussion around this, certainly in the groups that I’m involved with; and we know that there are greater discussions to be had in the next few months.
Dr Elisabeth Philipps
Nutritional Therapist
www.hartwellnutrition.co.uk
SOURCE: https://www.healtheuropa.eu/cbd-in-medicine-relief-and-regulation/99418/