Kai Medical Laboratory, a state-of-the-art diagnostics laboratory in Dallas, was acquired by Empower Clinics on October 6, 2020 to further advance the Company’s COVID-19 national testing programs for enterprise clients, including movie and television studios, businesses and colleges
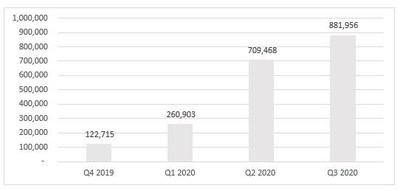
- KAI testing volume of 1,375 units in October represents a sequential increase of 763% over testing volume in September of 180 units.
- Moreover, the increase comes from only a partial month of control under Empower given the acquisition of KAI was only completed on October 6, 2020.
- Finally, given the very recent closing of the acquisition, the record October results were achieved without the benefit of Empower’s planned sales, marketing and rollout plans for KAI.
VANCOUVER, BC / November 13, 2020 / EMPOWER CLINICS INC. (CSE:CBDT)(Frankfurt:8EC)(OTCQB:EPWCF) (“Empower” or the “Company“) an integrated healthcare company serving a database of 165,000 patients through clinics in the southwest United States, a telemedicine platform and medical diagnostics laboratory, is pleased to announce that Kai Medical Laboratory (“KAI”) has achieved record testing volume for the month of October with 1,375 units processed.
RECORD ACHIEVED IN PARTIAL FIRST MONTH UNDER EMPOWER CLINICS
KAI testing volume of 1,375 units in October represents a sequential increase of 763% over testing volume in September of 180 units. Moreover, the increase comes from only a partial month of control under Empower given the acquisition of KAI was only completed on October 6, 2020. Finally, given the very recent closing of the acquisition, the record October results were achieved without the benefit of Empower’s planned sales, marketing and rollout plans for KAI.
Empower Clinics Chairman and CEO, Steven McAuley, stated “The magnitude of KAI’s growth and success in October cannot be overstated as an example of what is to come. This is especially true given the fact we have yet to fully implement the sales and marketing strategies planned for KAI as we expand our national testing programs for enterprise level clients across America. Though we are very pleased with the results achieved in October, the testing volume of 1,375 units represents a fraction of the lab’s capacity which is capable of achieving 4,000 COVID-19 RT-PCR tests per day. It is our intention to continue ramping up growth significantly through 2020 and well into 2021.”
GROWTH STRATEGIES & NUMEROUS NEW COMMERCIAL TESTING CONTRACTS
The Company is now working through integration and implementing aggressive growth strategies including servicing new COVID-19 testing contracts for the film & television industry, banks, restaurants, tourism and supporting the Sun Valley Health COVID-19 RT-PCR and rapid antibody testing programs in the state of Arizona.
To this end, on October 7th, Empower announced it had commenced COVID-19 RT-PCR tests for a major studio film & television production. On October 19th, Empower announced a partnership with Loop Insights to provide the first ever “travel bubble” solution for the global travel industry.
Finally, Empower has signed numerous new commercial testing contracts, the details of which will be provided in subsequent press releases.
To further assist with COVID-19 testing, Kai Medical Laboratory has also developed two key programs in Texas and Arizona. The first program is a direct-to-consumer program that leverages the ability of various healthcare providers to order and administer both the RT-PCR test and the Antibody test. This increases the ability of the general population to be tested, in certain circumstances. The second is an Employer COVID-19 Compliance Program (ECCP) for business owners and employer groups to enable them to test and monitor their employees.
Texas program https://www.testtexasnow.com
Arizona program https://www.covidtest2u.com
The combination of all this commercial activity since the October 6th acquisition provides the Company with the confidence necessary to support the expectation of much further growth in the months to come.
COVID-19 RT-PCR TESTING IS THE GOLD STANDARD THAT ALLOWS EMPOWER TO ROLL OUT NATIONAL PHASE 4 TESTING PROGRAMS
KAI Medical Laboratory operates a high-complexity CLIA and COLA accredited laboratory that provides reliable and accurate testing solutions to hospitals, medical clinics, pharmacies, and employer groups. KAI has taken an active role in COVID-19 testing, battling the pandemic through RT-PCR testing and serology testing with the capacity to process 4,000 RT-PCR test specimens per day. While the RT-PCR test identifies if a patient has an active virus, the serology or antibody test detects if a patient has previously been exposed to the virus. Both of these test results are vital to managing outbreaks and the potential spread of coronavirus.
As a result of this capability, Empower is now able to expand phase four of its COVID-19 testing rollout which was first announced on April 27, 2020 beginning with testing in-clinic testing (Phase 1) and culminating with a nationwide roll-out across the United States (Phase 4). Phase 4 allows Empower to service enterprise level clients, including movie and television studios that require reliable, accurate, fast and mass batch testing capabilities in order to resume production in a safe and compliant manner.
The Company has also granted an aggregate of 1,079,666 stock options (each, an “Option“) to certain key employees of the Company in accordance with the Company’s stock option plan priced at $0.05 CAD and 1,500,000 Options priced at $0.08 CAD to an investor relations service provider.
This press release is available on the Empower Clinics Verified Forum on AGORACOM for shareholder discussion, questions and engagement with management https://agoracom.com/ir/EmpowerClinics
ABOUT EMPOWER
Empower is creating a network of physicians and practitioners who integrate to serve patient needs, in-clinic, through telemedicine, and with decentralized mobile delivery. A simplified, streamlined care model bringing key attributes of the healthcare supply chain together, always focused on patient experience. The Company provides COVID-19 testing services to consumers and businesses as part of a four-phased nationwide testing initiative in the United States. Empower recently acquired Kai Medical Laboratory, LLC as a wholly owned subsidiary with large-scale testing capability.
ON BEHALF OF THE BOARD OF DIRECTORS:
Steven McAuley
Chief Executive Officer
CONTACTS:
Investors: Dustin Klein
Director
[email protected]
720-352-1398
Investors: Steven McAuley
CEO
[email protected]
604-789-2146
DISCLAIMER FOR FORWARD-LOOKING STATEMENTS
This news release contains certain “forward-looking statements” or “forward-looking information” (collectively “forward looking statements”) within the meaning of applicable Canadian securities laws. All statements, other than statements of historical fact, are forward-looking statements and are based on expectations, estimates and projections as at the date of this news release. Forward-looking statements can frequently be identified by words such as “plans”, “continues”, “expects”, “projects”, “intends”, “believes”, “anticipates”, “estimates”, “may”, “will”, “potential”, “proposed” and other similar words, or information that certain events or conditions “may” or “will” occur. Forward-looking statements in this news release include, but are not limited to, statements regarding: the expected benefits to the Company and its shareholders as a result of the acquisition of Kai Medical Laboratory; the transaction terms; the expected number of clinics and patients following the closing; the future potential success of Kai Medical Laboratory, Sun Valley’s franchise model; the anticipated date of closing of the acquisition and the occurrence thereof; and that the Company will be positioned to be a market-leading service provider for complex patient requirements in 2020 and beyond. Such statements are only projections, are based on assumptions known to management at this time, and are subject to risks and uncertainties that may cause actual results, performance or developments to differ materially from those contained in the forward-looking statements, including: that the Kai Medical Laboratory acquisition may not be completed on the terms expected or at all; that the Company’s products may not work as expected; that the Company may not be able to expand COVID-19 testing; that legislative changes may have an adverse effect on the Company’s business and product development; that the Momentum Health acquisition may not be completed on the terms expected or at all; that the Company may not be able to obtain adequate financing to pursue its business plan; general business, economic, competitive, political and social uncertainties; failure to obtain any necessary approvals in connection with the proposed transaction; and other factors beyond the Company’s control. No assurance can be given that any of the events anticipated by the forward-looking statements will occur or, if they do occur, what benefits the Company will obtain from them. Readers are cautioned not to place undue reliance on the forward-looking statements in this release, which are qualified in their entirety by these cautionary statements. The Company is under no obligation, and expressly disclaims any intention or obligation, to update or revise any forward-looking statements in this release, whether as a result of new information, future events or otherwise, except as expressly required by applicable laws.
SOURCE: Empower Clinics Inc.