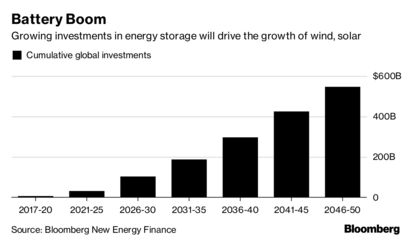
- The Swanson maiden mineral resource estimate for a combined pit-constrained and underground scenario is as follows:
- A pit-constrained Indicated resource of 90,319 ounces and Inferred resource of 941 ounces, and an underground Indicated resource of 7,732 ounces and Inferred resource of 5,975 ounces.
- The advantageous location of the Swanson deposit, some 100 metres from a railway track, provides easy access to the Beacon mill.
- The Corporation holds a mining lease on the Swanson property, enabling it to put the deposit into production more rapidly.
- Monarques Gold continues to increase its combined Measured and Indicated resources to more than 3.1 million ounces of gold (see table 3 at the end of press release).
MONTREAL, June 20, 2018 /CNW/ – MONARQUES GOLD CORPORATION (“Monarques”, “Monarques Gold” or the “Corporation”) (TSX-V:MQR) (OTCMKTS:MRQRF) (FRANKFURT:MR7) is pleased to report the results of a mineral resource estimate for its Swanson gold project, 65 kilometres north from its wholly-owned Beacon mill and 12 kilometres northeast of Barraute, Quebec. The CN railway line crosses the property, some 100 metres north of the Swanson deposit. Monarques acquired a 100% interest in the Swanson and McKenzie Break properties from Agnico Eagle Mines Limited (NYSE:AEM, TSX:AEM) (see press release dated December 21, 2017). The resource estimate was prepared by Christine Beausoleil, P.Geo. and Alain Carrier, P.Geo., M.Sc., of InnovExplo Inc., both qualified and independent persons as defined by NI 43-101. The effective date of the estimate is June 15, 2018.
“The great advantage of the Swanson project is that the railway track is directly on the property, which allows easy access to both our mills,” said Jean-Marc Lacoste, President and Chief Executive Officer of Monarques. “Much like for the McKenzie Break project, we are very pleased to have a pit-constrained resource for the Swanson project (see figure), as this could also mean additional feed for the Beacon mill. Â In addition, Monarques holds a mining lease on the Swanson property, which could allow us to put the deposit into production more quickly. Our new strategy of targeting pit-constrained resources from our McKenzie Break and Swanson properties, together with the reopening of our Beacon Mill at the end of the year, could be a winning solution for all three projects.”
The 2018 maiden mineral resource estimate was prepared using Leapfrog GEO and GEOVIA GEMS software. Leapfrog was used for 3D modelling of the four mineralized zones while GEMS was used for grade estimation and block modelling. Statistical studies were done using Snowden Supervisor and Microsoft Excel software. The estimate was performed using 3D block modelling with the Ordinary Kriging interpolation method.
The diamond drilling database contains the results of 146 surface and 63 underground drill holes provided by Monarques. Basic univariate statistics were performed on datasets of individual raw gold assays for each zone and for the dilution envelope. The capping (30 g/t Au) was applied on raw assays before compositing at 1.5 m.
The estimate is reported for a potential scenario combining pit-constrained and underground resources at a cut-off grade of 0.8 g/t Au (pit constrained) and 2.7 g/t Au (underground). The cut-off grades were calculated using a gold price of USD1,296/oz, a CAD:USD exchange rate of 1.28 and the following parameters: (a) pit-constrained scenario: mining cost CAD4.94/t, processing cost CAD27.00/t, General and administrative CAD4.00/t, pit slope of 50 degrees used during Whittle optimization; (b) underground scenario: mining cost CAD90.00/t, processing cost CAD27.00/t, General and administrative CAD10.00/t.
The Swanson project mineral resource estimate for a potential scenario combining pit-constrained and underground resources at cut-off grades of 0.8 g/t Au (pit-constrained) and 2.7 g/t Au (underground) is summarized in the following table 1, whereas table 2 shows the sensitivity analysis of the Swanson maiden mineral resource estimate for the pit-constrained scenario.
Table 1 – Swanson Maiden Mineral Resource Estimate for a combined pit-constrained and underground scenario at a cut-off grade of 0.8 g/t Au (in pit) and 2.7 g/t Au (underground)
|
Indicated Resource |
Inferred Resource |
|||||
|
Zone |
Tonnage |
Grade |
Ounces |
Tonnage |
Grade |
Ounces |
|
Pit-constrained  |
1,568,000 |
1.79 |
90,319 |
12,000 |
2.44 |
941 |
|
Underground  |
75,000 |
3.21 |
7,732 |
60,000 |
3.10 |
5,975 |
|
Total |
1,643,000 |
1.86 |
98,051 |
72,000 |
2.99 |
6,917 |
|
Notes to the mineral resource table: |
|
|
• |
These mineral resources are not mineral reserves, as they do not have demonstrated economic viability. |
|
• |
The 2014 CIM definitions and guidelines for mineral resources have been followed. |
|
• |
Results are presented in situ and undiluted and considered to have reasonable prospects for economic extraction. |
|
• |
The estimation encompasses four zones with a minimum true thickness of 2.5 m using the grade of the adjacent material when assayed, or a value of zero when not assayed. |
|
• |
A high-grade capping of 30 g/t Au (4 g/t Au for the dilution envelope) was applied to assay grades prior to compositing grade for interpolation using an Ordinary Kriging interpolation method, based on 1.5 m composite for block size of 3 m x 3 m x 3 m. |
|
• |
Bulk density values were applied on the following lithological basis (g/cm3): I2 = 2.78; I4O, V3, V4 = 2.90, and OVB = 1.5. |
|
• |
The number of metric tons was rounded to the nearest thousand and the metal contents are presented in troy ounces (tonne x grade / 31.10348). |
|
• |
InnovExplo is not aware of any known environmental, permitting, legal, title-related, taxation, socio-political or marketing issues, or any other relevant issue not reported in this Technical Report that could materially affect the mineral resource estimate. |
Table 2 – Sensitivity analysis of the Swanson Maiden Mineral Resource Estimate for the pit-constrained scenario
|
Indicated Resource |
Inferred Resource |
||||||
|
Cut off |
Au (g/t) |
Tonnes |
Ounces |
Cut off |
Au (g/t) |
Tonnes |
Ounces |
|
0.6 |
1.65 |
1,798,000 |
95,499 |
0.6 |
2.22 |
14,000 |
997 |
|
0.7 |
1.72 |
1,685,000 |
93,157 |
0.7 |
2.28 |
13,000 |
952 |
|
0.8 |
1.79 |
1,568,000 |
90,319 |
0.8 |
2.44 |
12,000 |
941 |
|
0.9 |
1.87 |
1,453,000 |
87,173 |
0.9 |
2.56 |
11,000 |
907 |
|
1 |
1.94 |
1,338,000 |
83,651 |
1 |
2.66 |
10,000 |
856 |
The NI 43-101 technical report will be delivered and filed on SEDAR within the next 45 days.
The technical and scientific content of this press release has been reviewed and approved by Marc-André Lavergne, Eng., the Corporation’s qualified person and by Christine Beausoleil, P.Geo. and Alain Carrier, P.Geo., M.Sc. of InnovExplo Inc., all of whom are qualified persons as defined by NI 43-101.
ABOUT MONARQUES GOLD CORPORATION
Monarques Gold Corporation (TSX.V:MQR) is an emerging gold producer focused on pursuing growth through its large portfolio of high-quality projects in the Abitibi mining camp in Quebec, Canada. The Corporation currently owns close to 300 km² of gold properties (see map), including the Beaufor Mine, the Croinor Gold (see video), Wasamac, McKenzie Break and Swanson advanced projects, and the Camflo and Beacon mills, as well as six promising exploration projects. It also offers custom milling services out of its 1,600 tonne-per-day Camflo mill. Monarques enjoys a strong financial position and has more than 150 skilled employees who oversee its operating, development and exploration activities.
Forward-Looking Statements
The forward-looking statements in this press release involve known and unknown risks, uncertainties and other factors that may cause Monarques’ actual results, performance and achievements to be materially different from the results, performance or achievements expressed or implied therein. Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this press release.
Table 3 – Monarques Gold Measured and Indicated Resources
|
Tonnes |
Grade |
Ounces |
|
|
Wasamac property1 |
|||
|
Measured Resources |
3.99 million |
2.52 |
323,300 |
|
Indicated Resources |
25.87 million |
2.72 |
2,264,500 |
|
Total Measured & Indicated Resources |
29.86 million |
2.70 |
2,587,900 |
|
Beaufor Mine2 |
|||
|
Measured Resources |
74,400 |
6.71 |
16,100 |
|
Indicated Resources |
271,700 |
7.93 |
69,300 |
|
Total Measured & Indicated Resources |
346,200 |
7.67 |
85,400 |
|
Croinor Gold Mine3 |
|||
|
Measured Resources |
80,100 |
8.44 |
21,700 |
|
Indicated Resources |
724,500 |
9.20 |
214,300 |
|
Total Measured & Indicated Resources |
804,600 |
9.12 |
236,000 |
|
Swanson property4 |
|||
|
Indicated Resources |
1,643,000 |
1.86 |
98,051 |
|
McKenzie Break property5 |
|||
|
Pit Constrained |
|||
|
Indicated Resources |
939,860 |
1.59 |
48,133 |
|
Underground |
|||
|
Indicated Resources |
281,739 |
5.90 |
53,448 |
|
Simkar Gold property6 |
|||
|
Measured Resources |
33,570 |
4.71 |
5,079 |
|
Indicated Resources |
208,470 |
5.66 |
37,905 |
|
Total Measured & Indicated Resources |
242,040 |
5.52 |
42,984 |
|
TOTAL |
|||
|
Measured & Indicated Resources |
3,151,916 |
||
|
1 Source: Technical Report on the Wasamac Project, Rouyn-Noranda, Québec, Canada, Tudorel Ciuculescu, M.Sc., P.Geo., October 25, 2017, Roscoe Postle Associates Inc. |
|||
|
2 Source: NI-43-101 Technical Report on the Mineral Resource and Mineral Reserve Estimates of the Beaufor Mine as at September 30, 2017, Val-d’Or, Québec, Canada, Carl Pelletier, P. Geo. and Laurent Roy, Eng. |
|||
|
3 Source: Monarques prefeasibility study (January 19, 2018) and resource estimate (January 8, 2016) 4 Source: NI 43â€101 Technical Report on the Swanson Project, June 15, 2018, Christine Beausoleil, P.Geo. and Alain Carrier, P.Geo., M.Sc. of InnovExplo Inc. 5 Source: NI 43â€101 Technical Report on the McKenzie Break Project, April 17, 2018, Alain-Jean Beauregard, P.Geo., and Daniel Gaudreault, Eng., of Geologica Groupe-Conseil Inc., and Christian D’Amours, P.Geo., of 6 Source: MRB et Associés (January 2015) |
|||
![]() View original content with multimedia:http://www.prnewswire.com/news-releases/monarques-gold-estimates-pit-constrained-resource-on-its-swanson-gold-project-300669001.html
View original content with multimedia:http://www.prnewswire.com/news-releases/monarques-gold-estimates-pit-constrained-resource-on-its-swanson-gold-project-300669001.html
SOURCE Monarques Gold Corporation
 View original content with multimedia: http://www.newswire.ca/en/releases/archive/June2018/20/c8059.html
View original content with multimedia: http://www.newswire.ca/en/releases/archive/June2018/20/c8059.html
Jean-Marc Lacoste, President and Chief Executive Officer, 1-888-994-4465, [email protected], www.monarquesgold.com; Elisabeth Tremblay, Senior Geologist – Communications Specialist, 1-888-994-4465, [email protected], www.monarquesgold.comCopyright CNW Group 2018