tZERO – AGORACOM Partnership Establishes The First Institutional-Grade Framework Specifically Designed For Tokenizing Small- and Mid-Cap Public Company Assets
NEW YORK, NY – January 22, 2026 AGORACOM RWA DBX, the real-world asset (RWA) tokenization initiative of AGORACOM, and tZERO Group Inc, a leading innovator in blockchain-powered multi-asset infrastructure, today announced a strategic partnership to support the compliant tokenization of RWAs held by small- and mid-cap public companies.
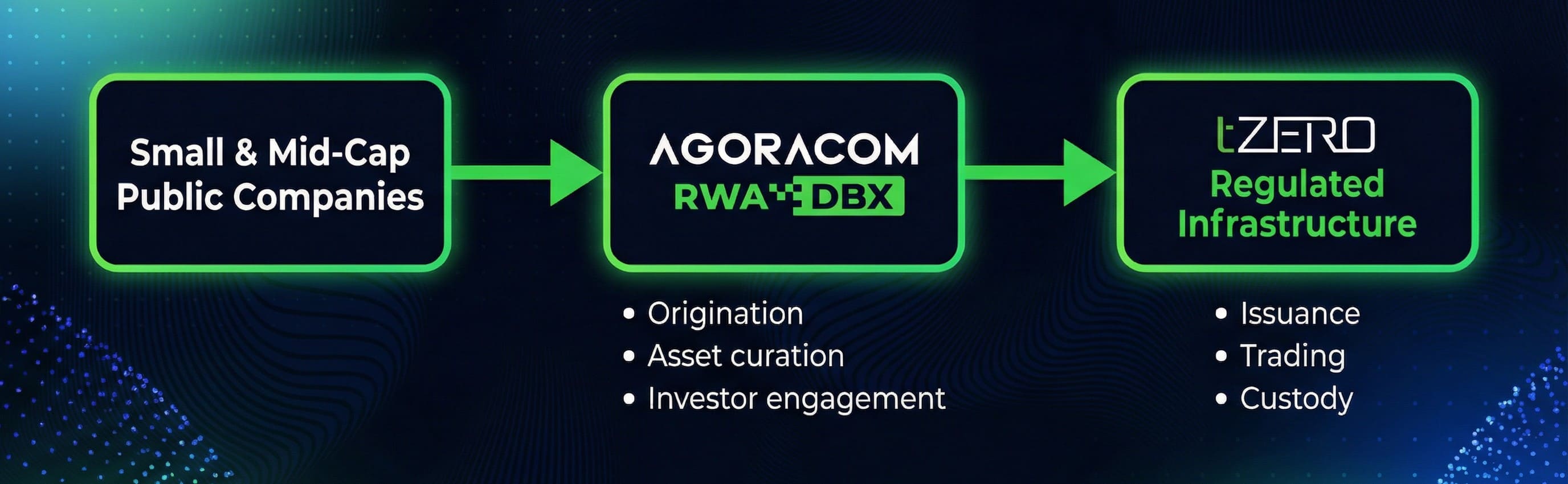
The partnership formally aligns AGORACOM’s issuer origination, asset curation, and investor engagement platform with tZERO’s institutional-grade capital markets infrastructure, creating a structured pathway for small-cap public companies to tokenize real-world assets that reach global investors within established regulatory frameworks.
tZERO: Regulated Capital Markets Infrastructure for the Tokenized Economy
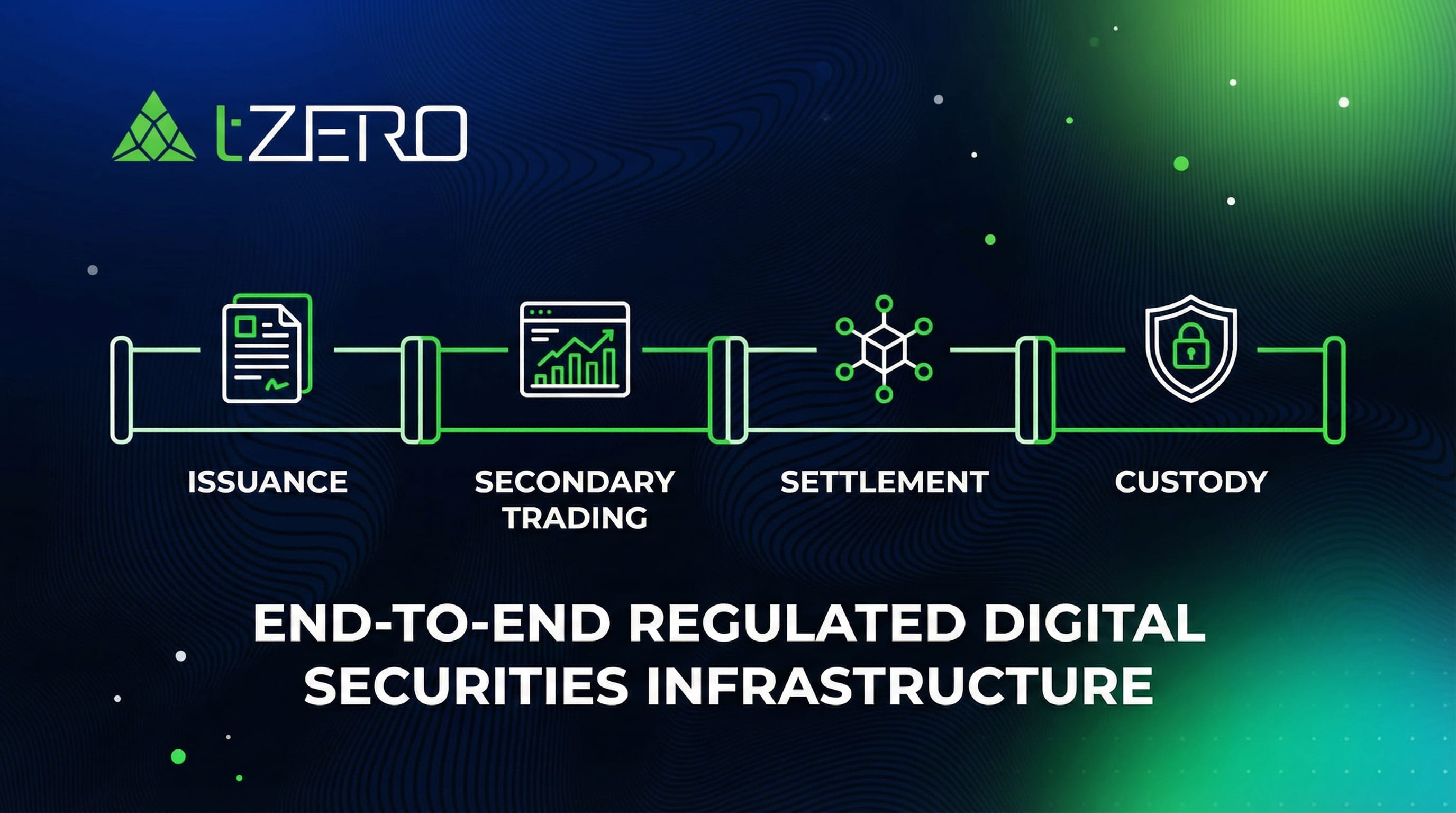
tZERO operates one of the most comprehensive regulated digital securities infrastructures in the market today, providing end-to-end capabilities across issuance, secondary trading, settlement, and on-chain custody of tokenized securities. Built to integrate with traditional capital markets, tZERO’s platform is designed to support compliant digital representations of securities and asset-backed instruments across multiple asset classes and jurisdictions.
Through this partnership, tZERO will serve as the regulated infrastructure backbonewithin the AGORACOM RWA DBX ecosystem, supporting qualifying offerings with capital markets rigor, operational discipline, and regulatory oversight consistent with institutional standards.
“One of the challenges the tokenization space has been dealing with is attacking the historically illiquid, niche and opaque asset classes first. Public companies and their real-world asset bases – supported by existing disclosure market analysis and investor understanding – solve that issue. And public companies need tokenization models that align with existing disclosure, governance, and regulatory requirements,” said Alan Konevsky, Chief Executive Officer at tZERO. “This partnership is designed to extend familiar capital markets principles into tokenization form, allowing issuers to evaluate tokenization of assets, intellectual property and revenue streams as a structured, compliant financing tool to unlock liquidity and raise non-dilutive capital.”
tZERO is one of only two firms in the United States approved as a Special Purpose Broker-Dealer, allowing it to custody digital securities within a regulated broker-dealer framework, with their infrastructure already being utilized across a growing set of regulated digital securities use cases, including:
- Multiple live and completed regulated digital securities offerings, spanning private and public company issuers
- Tens of millions of digital securities traded through regulated secondary market infrastructure
- Operational issuance, trading, settlement, and custody under a U.S. broker-dealer framework
- Active engagement with institutional issuers, investors, and market participants seeking compliant tokenization pathways
- Years of direct regulatory engagement and approvals, reflecting sustained investment in compliance-first market infrastructure
These real-world deployments underscore tZERO’s role as a production-grade capital markets platform and provide the foundation required to support asset tokenization initiatives at institutional standards.
AGORACOM RWA DBX: A New Category Of Real-World Assets From Companies That Are Regulated
AGORACOM RWA DBX is pioneering a new RWA category by tokenizing the assets of small- and mid-cap public companies that are:
- Real Growth Assets
- Verified By Securities Filings
- Supported By Audited Financials
- Subject To Regulatory Oversight & Continuous Disclosure
Those assets, which have have historically lacked modern, institutional-grade pathways to capital formation and global investor visibility, include but are not limited to:
- Mineral and Resource Projects (Gold, Silver, Critical Minerals)
- Specialty Materials
- Energy Technologies
- Intellectual Property
- Fintech Platforms

AGORACOM and tZERO are addressing that gap by providing issuers with an effective, credible and institutional approach to tokenization, designed to expand financing and investor access options at scale while maintaining the regulatory rigor required by sophisticated market participants.
The partnership with tZERO provides the regulatory and operational foundation required to develop this category responsibly and at scale.
“This partnership is about giving small and mid-cap public companies better options,” said George Tsiolis, Founder of AGORACOM. “By combining AGORACOM RWA DBX’s issuer expertise and engagement capabilities with tZERO’s institutional-grade, regulated infrastructure, we are creating a serious, execution-ready framework for asset tokenization that sophisticated investors, funds and family offices around the world can trust.”
What This Partnership Enables for Small and Mid-Cap Issuers
This partnership establishes a clear, institutional-grade pathway for small and mid-cap public companies to evaluate and implement asset-level tokenization as a legitimate capital markets strategy.
Through AGORACOM RWA DBX, supported by tZERO’s regulated infrastructure, issuers can now approach tokenization with the same level of rigor, compliance and credibility expected in traditional capital markets. This includes a disciplined process for identifying suitable operating assets, structuring compliant digital securities and introducing those instruments to global investor markets through regulated channels.
For management teams, this means tokenization is no longer theoretical or experimental. It becomes a practical, execution-ready option – designed to complement existing public listings by adding a regulated, asset-backed financing lane that preserves disclosure standards, governance, and institutional trust.
The objective is not to replace public markets, but to expand the toolkit available to serious public companies seeking modern, non-dilutive ways to unlock asset value and broaden investor access.
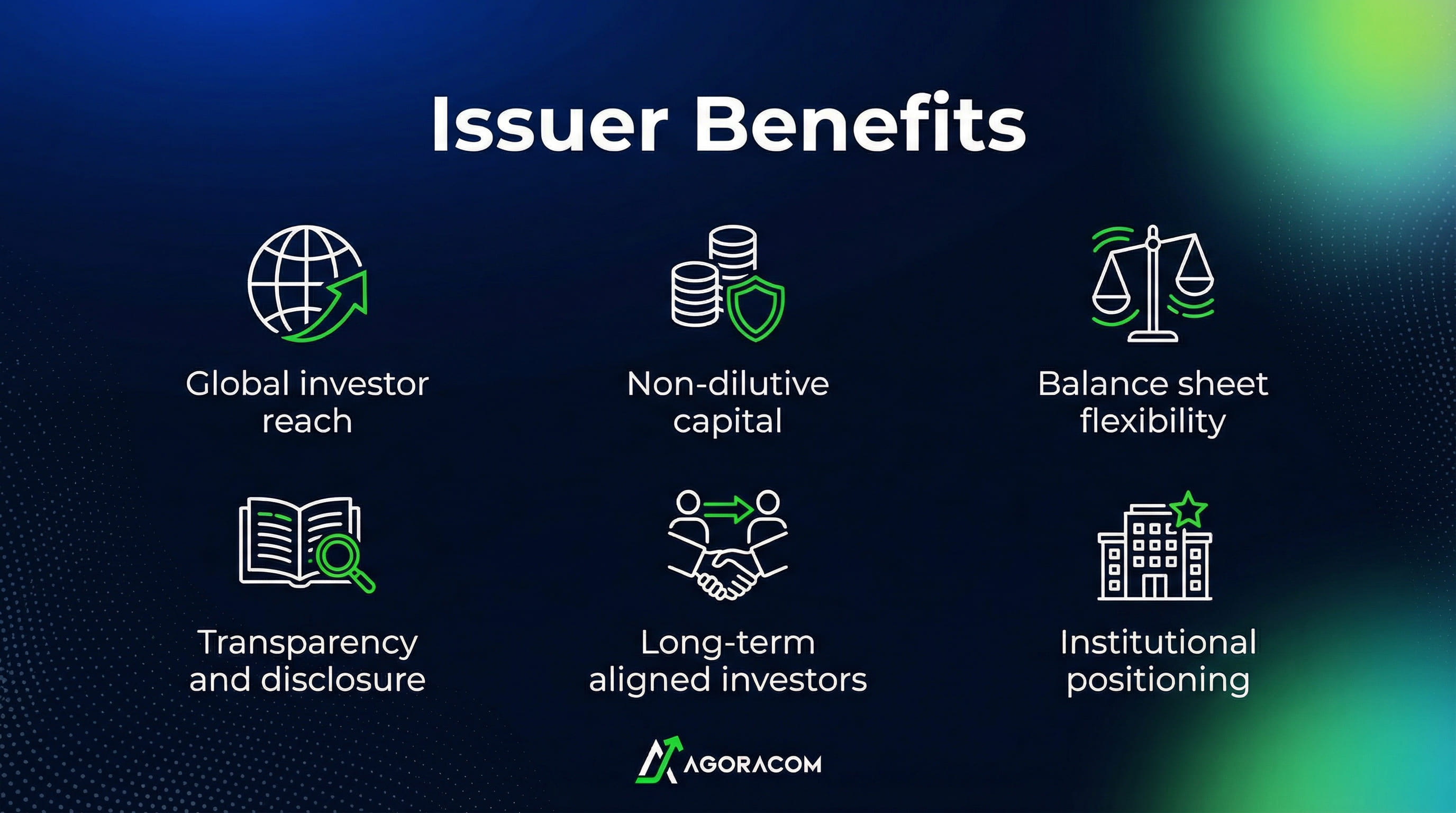
What This Means for Small and Mid-Cap Issuers
-
- Expand global investor reach by introducing asset-backed digital securities through regulated infrastructure accessible to institutional, family office, and sophisticated investors across multiple jurisdictions
- Unlock value from existing operating assets without relying exclusively on common share dilution, enabling capital formation strategies that are better aligned with long-term asset development
- Strengthen balance-sheet and financing optionality by creating new, compliant funding lanes that can sit alongside traditional equity and debt markets
- Increase investor understanding and confidence through transparent, asset-level structures supported by audited financials, public disclosure, and regulated custody and trading
- Build a more resilient shareholder base by engaging investors who are aligned with asset performance and long-term company fundamentals, rather than short-term trading dynamics
- Position the company as forward-looking and institution-ready, demonstrating governance discipline and strategic sophistication to boards, regulators, and capital providers
AGORACOM’s Role: Origination, Curation, and Engagement
AGORACOM RWA DBX sits at the center of the ecosystem as the category builder and origination platform, leveraging AGORACOM’s more than 25-year history working with small-cap public companies and investors.
AGORACOM RWA DBX is responsible for identifying and curating suitable issuers, supporting asset-level analysis, and delivering the education, communication, and investor-engagement infrastructure required for sustainable adoption. This includes moderated investor forums, digital content, and issuer-focused education designed to align market understanding with institutional expectations.
The initiative is already gaining momentum. AGORACOM has signed its first RWA letter of intent with a public company, hosted its inaugural RWA webcast with more than 200 small-cap CEOs and stakeholders, and announced ecosystem partnerships with Dubai Blockchain Center, BlockRidge, and Pegasus Fintech as it prepares for meaningful deal flow beginning in 2026 and accelerating through 2030.
Next Steps for Public Companies
AGORACOM RWA DBX is already working with a limited number of small and mid-cap public companies to evaluate asset-level tokenization opportunities within this newly established framework.
Management teams and boards interested in understanding whether their operating assets may be suitable for this approach are encouraged to engage in early exploratory discussions. These initial conversations are intended to assess asset characteristics, governance considerations, and strategic fit well before any formal structuring or execution decisions are made. Please visit our website https://agoracomrwa.com/

About tZERO
tZERO Group, Inc. (tZERO) and its broker-dealer subsidiaries provide an innovative liquidity platform for private companies and assets. We offer institutional-grade solutions for issuers looking to digitize their capital table through blockchain technology, and make such equity available for trading on an alternative trading system. tZERO, through its broker-dealer subsidiaries, democratizes access to private assets by providing a simple, automated, and efficient trading venue to broker-dealers, institutions, and investors. All technology services are offered through tZERO Technologies, LLC. For more information, please visit our website www.tZERO.com
About tZERO Digital Asset Securities, LLC
tZERO Digital Asset Securities, LLC is a broker-dealer registered with the SEC and a member of FINRA and SIPC. It is the broker-dealer custodian of all digital asset securities offered on tZERO’s online brokerage platform. More information about tZERO Digital Asset Securities may be found on FINRA’s BrokerCheck.
About tZERO Securities, LLC
tZERO Securities, LLC is a broker-dealer registered with the SEC and a member of FINRA and SIPC. It is the operator of the tZERO Securities ATS. More information about tZERO Securities may be found on FINRA’s BrokerCheck.
Forward-Looking Statements by tZero
This release contains forward-looking statements. In addition, from time to time, tZERO, its subsidiaries, or its representatives may make forward-looking statements orally or in writing. These forward-looking statements are based on expectations and projections about future events, which is derived from currently available information. Such forward-looking statements relate to future events or future performance, including financial performance and projections; growth in revenue and earnings; and business prospects and opportunities. You can identify forward-looking statements by those that are not historical in nature, particularly those that use terminology such as “may,” “should,” “expects,” “anticipates,” “contemplates,” “estimates,” “believes,” “plans,” “projected,” “predicts,” “potential,” or “hopes” or the negative of these or similar terms. In evaluating these forward-looking statements, you should consider various factors, including, without limitation: the ability of tZERO and its subsidiaries to change the direction; tZERO’s ability to keep pace with new technology and changing market needs; performance of individual transactions; regulatory developments and matters; and competition. These and other factors may cause actual results to differ materially from any forward-looking statement. Forward-looking statements are only predictions. The forward-looking events discussed in this release and other statements made from time to time by tZERO, its subsidiaries or their respective representatives, may not occur, and actual events and results may differ materially and are subject to risks, uncertainties and assumptions. tZERO, its subsidiaries, and its representatives are not obligated to publicly update or revise any forward-looking statement, whether as a result of uncertainties and assumptions, the forward-looking events discussed in this release and other statements made from time to time by tZERO, its subsidiaries or its representatives might not occur.
About AGORACOM RWA DBX
AGORACOM RWA DBX is a Dubai-based consulting agency that is focused on helping small and mid-cap public companies unlock growth capital by tokenizing real-world assets held in subsidiaries. Working with a network of leading independent legal, financial, and technical partners from around the world, the firm provides a fully coordinated process from structure and compliance to token issuance and listing on exchanges that reach primary and secondary markets. The tokenization program is designed to offer non-dilutive financing alternatives that preserve equity structure, increase asset liquidity, and open new access to global capital. AGORACOM RWA DBX does not provide virtual-asset services in or from the Emirate of Dubai and this announcement is not an offer to the public.
By focusing on listed issuers in North America that are fully regulated and audited by multiple securities regulators, AGORACOM RWA DBX is creating a brand new asset class in Real World Asset Tokenization that provides investors around the world with access to growth assets of emerging public companies that are fully verifiable and conservatively projected to be a $10 billion market by 2030, with the entire RWA market projected to reach $16 trillion. This new asset class represents a significant alternative for growth investors to current RWA Tokens which are largely limited to sovereign treasuries, mega real estate projects, stock of mega cap issuers and non-compliant or opaque tokens.
AGORACOM RWA DBX collaborates with AGORACOM, the pioneer of online investor relations and a digital marketing platform with a 27 year track record of serving 500 public companies and 9,000,000 investors.
Learn more at https://agoracomrwa.com/








