
- PFN Adds a 2nd Round of Staked Claims, at their River Valley Project
- These Newly Acquired Claims Expand Coverage of Internal and Footwall Targets
- PFN’s Total Landholding at River Valley Now Increased to 104.5 km2 or 10,454 ha
- PFN’s River Valley Project consists of a Primary Platinum Metal Deposit of 2.5 Moz Platinum Metals, in Near-Surface Measured and Indicated Resources
- The Project is within 100 road-kms of Sudbury and its Excellent Infrastructure and Support
- Fall Drill Program Underway
- New 100%-owned Li Division, with 5 Hard Rock Pegmatite Projects, in Manitoba and Lithium Brine Projects, in Nevada – Fall Surface Programs Underway
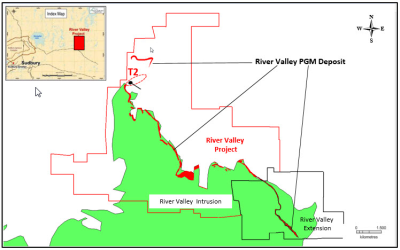
Vancouver, British Columbia / November 3, 2016 – Pacific North West Capital Corp. (“PFN” the “Company”) (TSXV: PFN; OTCQB: PAWEF; FSE: P7J) is pleased to announce staking of 14 New, Unpatented Mining Claims, adjacent to its River Valley PGM Project (RVP) and north of its Recently Expanded River Valley Extension Project (Figure 1). The River Valley Platinum Metal Group (PGM) Deposit, on RVP, has been targeted by many drill campaigns, since 2000, which have returned High Grade Assays, at Shallow Depths and led to several large mineral resource estimates and positive metallurgical studies (for details see PFN websitewww.PFNCapital.com). Collectively, the Newly Expanded RVP, plus the Recently Acquired and Expanded River Valley Extension Project (see:Press Release October 5th, 2016) now comprise a total landholding of 10,454 ha. This large landholding gives PFN a Strategic Land Position and district scale PGM potential, near Sudbury, home to one of the World’s Largest Nickel-Copper-PGM Mining and Metallurgical Complexes.
The Expanded RVP overlies more of the interior of the River Valley Intrusion, in addition to the basal contact area. PGM Exploration has focused on the basal contact, hosted main mineralized trend and a very large, near-surface mineral resource has been delineated. However, the internal portion of the River Valley Intrusion, has not been systematically explored, despite the presence of high-grade PGM grab samples, mineralization in sporadic drilling, and geophysical anomalies. Approximately $10M in assessment credits can be applied to the claims, within the RVP property, allowing PFN to Focus Exploration on the Highest Priority Targets. Exploration is Underway and a Drill Program began in early October; results will be released in the Coming Weeks and Months.
Click Image To View Full Size
Figure 1: Geological map showing the location of PFN’s newly expanded River Valley and River Valley Extension Projects, within 100 road-kms of Sudbury (Ontario). Note that the River Valley PGM deposit extends for a strike length of 16 km, is up to 200 metres wide and is open at depth.
About PFN’s Platinum Group Metals Division
River Valley is Canada’s Largest Undeveloped Primary PGM Deposit.
Achievements to date and Future Plans, for River Valley, are outlined below as follows:
- 1.PFN currently has 100% ownership in the River Valley Project, subject to a 3% NSR, with Options to Buy Down
- 2.Completed Exploration and Development Programs, on the River Valley Property:
Include more than 600 holes drilled, since year 2000, and several Mineral Resource Estimates and Metallurgical Studies
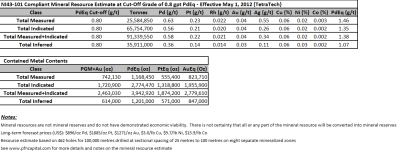
- 3.Results for the current (2012) Mineral Resource Estimate are below
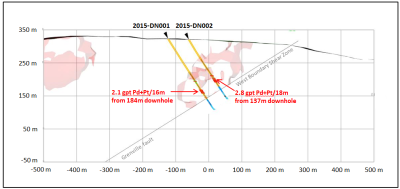
- 4.2015 Drill Program confirms New High Grade T2 Discovery
- 5.Exploration and Development Plans outlined for 2016
- 6.Ongoing Strategic Partner Search for River Valley Project
- 7.Results for the current Mineral Resource Estimate are summarized below:
– Prepared by Tetra Tech (Wardrop)
– High Confidence: Measured plus Indicated = 72% of total
– Reported on PdEq basis: Pd=40% & Pt=20% of the Payable Metals
– Pd to Pt ratio = 2.5:1; Cu to Ni ratio = 3:1
– High Grade Potential: particularly in the north part of the River Valley Deposit
Resources under Evaluation for Development Potential as Open Pit Mining Operation
Click Image To View Full Size

Click Image To View Full Size
- 8.Results for the 2015 Discovery Drill Program on the T2 Target are as follows:
-Drill hole intercepts much higher than the average grade, of current Mineral Resource Estimate
-Possible New Mineralized Zone at the north end of the River Valley Deposit
-Show potential to take the River Valley PGM Project in a New Direction
-More drilling required and field crews have completed ground-proofing targets, to better plan Fall and Winter Drill Programs
- 9. Exploration and Development Plans for 2016:
-Mineral Prospecting and Geological Mapping on surface: In Progress
-Drill Programs targeted to add more, higher grade: Drilling Started October 2016
-Geological Interpretation and 2D/3D Modelling of all Drill and Surface Results
-Ongoing Strategic Partner Search for River Valley
Click Image To View Full Size
About PFN’s Lithium Division
The Company’s Lithium Division will focus on the Discovery, Acquisition, Exploration and Development of Lithium Projects in Canada. In the United States, the Company will use its wholly owned U.S.A subsidiary to Acquire and Develop Projects, in Active Mining Camps, in Nevada, Arizona and California.
Management believes that these New Age Metals, Lithium, PGMs and Rare Earths, have robust macro trends with surging demands and limited supply. Going forward, this New Division will Explore for the Minerals needed to fuel the demand for Energy Storage and other Core 21st Century Technologies.
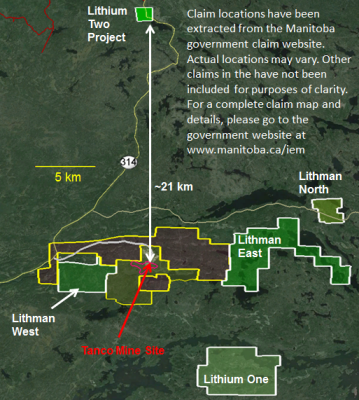
The Company has a Growing Portfolio of Lithium Projects: The Clayton Valley Forks Li Project, in Nevada, is a recent Lithium Brine Project, Acquired by the Company (see PFN News Releases: April 25th, 2016 and May 9th, 2016). The Company also has several Hard Rock Lithium Projects in Canada: To date the Company has Acquired 5 Hard Rock Lithium Projects, in the Winnipeg River Pegmatite Field, in SE Manitoba(see PFN News Releases: April 21st, 2016;May24th, 2016; June 15th, 2016; July 5th, 2016 and July 21st, 2016). This Pegmatite Field hosts the Giant Tanco Pegmatite Mine that has been mined for Tantalum, Cesium and Spodumene (one of the Primary Lithium Ore Minerals) in varying capacities, since 1969. Today, the Tanco Mine is focused on the Mining and Production of Cesium Formate, a drilling fluid for the petroleum industry. PFN’s Li Projects are strategically situated to Further Explore this Pegmatite Field.Presently, the Company is the Largest Claim Holder in the Winnipeg River Pegmatite Field.
Lithium and Platinum Group Metal Prices have improved drastically, in recent months. Lithium supplies remain in deficit, relative to their demand. Both Metals Groups are used for the expanding worldwide automobile industry (conventional and electric). In the case of PGM’s, demand is increasing for Autocatalysts, a key component for reducing toxic emissions, for automotive, gasoline and diesel engines. Regarding Lithium, there is an ever increasing demand for batteries in cellphones, laptops, electric cars, solar storage, wireless charging and renewable energy products.
Click Image To View Full Size
Figure 3: PFN’s 5 New Lithium Projects in Manitoba, Surrounding Tanco Mine
Figure 4: Company Claim Blocks in the Clayton Valley area of Nevada
(Figure 4 is a Company-made composite and not intended for redistribution.
The Company accepts no responsibility for the accuracy of these claim blocks, other than the claim block associated with the Clayton Valley Forks Li Project)
Clayton Valley is located in Esmeralda County, Nevada, host to the Albemarle Corporation’s Silver Peak Lithium Mine and Brine Processing Operations. The mine has been in operation since 1967 and remains the only Brine-Based Lithium Producer in North America. The New Project Acquisition, in Nevada, provides the Company a Project, in an area that is well known for its Lithium Carbonate Production. Clayton Valley is a Centralized Location in Nevada, with Highway Access, Power Infrastructure, Water and Local Labor.
The company’s new Lithium Brine Project will be approximately 3.5 hours away from Tesla’s Gigafactory, which has a planned annual Lithium-ion battery production capacity of 35 gigawatt-hours per year, by 2020. The CV West Li Project is located approximately 3 hours north of the Faraday Electric Car Factory, to be operated in Las Vegas, Nevada.
Clayton Valley is one of the few locations globally, known to contain Commercial-Grade Lithium-Enriched Brines.
QUALIFIED PERSON
The contents contained herein, which relate to Exploration Results or Mineral Resources, is based on information compiled, reviewed or prepared by Dr. Bill Stone, Principal Consulting Geoscientist, for Pacific North West Capital. Dr. Stone is a Qualified Person, as defined by National Instrument 43-101 and has reviewed and approved the technical content.
On behalf of the Board of Directors
“Harry Barr”
Harry G. Barr
Chairman and CEO
Neither the TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Cautionary Note Regarding Forward Looking Statements: This release contains forward-looking statements that involve risks and uncertainties. These statements may differ materially from actual future events or results and are based on current expectations or beliefs. For this purpose, statements of historical fact may be deemed to be forward-looking statements. In addition, forward-looking statements include statements in which the Company uses words such as “continue”, “efforts”, “expect”, “believe”, “anticipate”, “confident”, “intend”, “strategy”, “plan”, “will”, “estimate”, “project”, “goal”, “target”, “prospects”, “optimistic” or similar expressions. These statements by their nature involve risks and uncertainties, and actual results may differ materially depending on a variety of important factors, including, among others, the Company’s ability and continuation of efforts to timely and completely make available adequate current public information, additional or different regulatory and legal requirements and restrictions that may be imposed, and other factors as may be discussed in the documents filed by the Company on SEDAR (www.sedar.com), including the most recent reports that identify important risk factors that could cause actual results to differ from those contained in the forward-looking statements. The Company does not undertake any obligation to review or confirm analysts’ expectations or estimates or to release publicly any revisions to any forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. Investors should not place undue reliance on forward-looking statements.




















